H2 & Fuel Cells
In the production systems for the next-generation energy source "hydrogen" and the fuel cell systems that generate electricity through the chemical reaction of hydrogen with oxygen in the air, ion-conducting polymers are used that transport protons (H+) and anions (OH-) at high speed. For further enhancement of these systems' performance, not only high ion conductivity but also high chemical and mechanical stability are required.
Ion Conductance & Chemical Properties
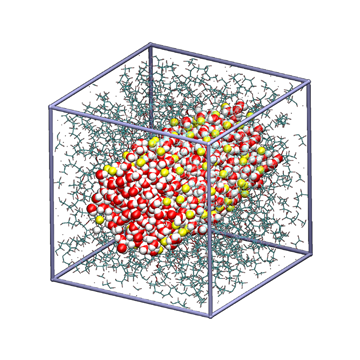
The transport of protons (H+) and anions (OH-) requires consideration of a complex mechanism involving chemical reactions with water molecules, known as the Grotthuss mechanism, unlike other ions. Moreover, these ions move through nanoscale water domains formed within the electrolyte membrane, making it difficult to understand the transport phenomena through experiments or macroscale analysis. Therefore, in this research, we use a reactive MD model developed based on quantum chemical calculations to clarify the correlation between the water domain structure and the ion conduction mechanism. We also evaluate the chemical stability of the polymer structure from the perspectives of oxidation radical resistance and alkali durability.
Relevent Papers
- T. Mabuchi*, “Revealing the anticorrelation behavior mechanism between the Grotthuss and vehicular diffusions for proton transport in concentrated acid solutions”, Journal of Physical Chemistry B, 126, 3319-3326 (2022).
- T. Mabuchi* and T. Tokumasu, “Effects of Water Nanochannel Diameter on Proton Transport in Proton-Exchange Membranes”, Journal of Polymer Science Part B: Polymer Physics, 57, 13, 867-878 (2019). (This article was selected for the cover paper)
- T. Mabuchi* and T. Tokumasu, “Relationship between Proton Transport and Morphology of Perfluorosulfonic Acid Membranes: A Reactive Molecular Dynamics Approach”, Journal of Physical Chemistry B, 122, 5922-5932 (2018).
- T. Mabuchi* and T. Tokumasu, “Dependence of electroosmosis on polymer structure in proton exchange membranes”, Mechanical Engineering Journal, Vol. 4, No. 5, 17-00054 (2017).
- T. Mabuchi*, A. Fukushima, and T. Tokumasu, “A Modified Two-State Empirical Valence Bond Model for Proton Transport in Aqueous Solutions”, Journal of Chemical Physics, 143, 014501 (2015).
Higher-Order Morphology & Mechanical Properties
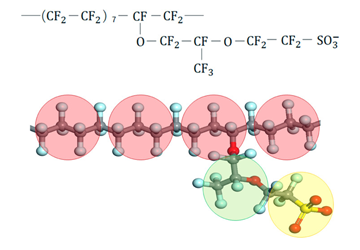
Polymers self-assemble and self-organize due to intramolecular and intermolecular interactions, forming various higher-order structures through molecular assemblies. These structures are significantly related to the properties of polymer materials; thus, controlling the micro-phase-separated structures, including nanoscale water domains that constitute the polymer materials, is essential for controlling the properties of polymer materials. In this research, using coarse-grained MD, where multiple atoms are treated as a single coarse-grained particle, we aim to elucidate the correlation between the primary and higher-order structures of constituent polymers and their properties.
Relevent Papers
- T. Mabuchi*, S. F. Huang, and T. Tokumasu, “Nafion Ionomer Dispersion in Mixtures of 1‐Propanol and Water Based on the Martini Coarse-Grained Model”, Journal of Polymer Science, 58, 487-499 (2020).
- W.Goncalves*, T. Mabuchi*, and T. Tokumasu, “Nucleation and Growth of Cavities in Hydrated Nafion Membranes under Tensile Strain: A Molecular Dynamics Study”, Journal of Physical Chemistry C, 123, 28958-28968 (2019).
- T. Mabuchi* and T. Tokumasu, “Effect of bound state of water on hydronium ion mobility in hydrated Nafion using molecular dynamics simulations”, Journal of Chemical Physics, 141, 104904 (2014).
Thin Films & Solution Processing
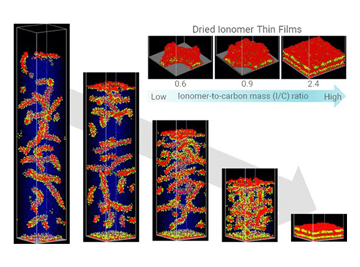
Ionomer thin films are advancing for use in a variety of applications, including fuel cells, water electrolysis, and polymer actuators/sensors. Due to the formation of thin films, the volume fraction of the interface region with the contact atmosphere or substances is high relative to the entire membrane. This necessitates a thorough understanding and control of the properties and interface structures with different materials, which are distinct from those of bulk polymers (micrometer order). Additionally, we are analyzing the aggregated structures of polymers in the dispersed liquids used in the film formation process.
Relevent Papers
- T. Mabuchi*, S. F. Huang, and T. Tokumasu, “Influence of Ionomer Loading and Substrate Wettability on the Morphology of Ionomer Thin Films Using Coarse-Grained Solvent Evaporation Simulations”, Macromolecules, 54, 115-125 (2021).
- T. Mabuchi*, S. F. Huang, and T. Tokumasu, “Dispersion of Nafion Ionomer Aggregates in 1-Propanol/Water Solutions: Effects of Ionomer Concentration, Alcohol Content, and Salt Addition”, Macromolecules, 53, 3273-3283 (2020).
- T. Mabuchi*, S. F. Huang, and T. Tokumasu, “Nafion Ionomer Dispersion in Mixtures of 1‐Propanol and Water Based on the Martini Coarse-Grained Model”, Journal of Polymer Science, 58, 487-499 (2020).
Secondary Batteries
Lithium-Ion Encapsulated Fullerene
Lithium-encapsulated fullerene (Li+@C60) is considered a new nanomaterial with unique characteristics not found in fullerene itself, such as very high ion conductivity. Its application to flexible organic electronic devices, such as energy storage devices and organic solar cells, is one of the potential uses. In this research, we are analyzing the dynamic behavior of Li+@C60 using MD simulations, aiming to develop its application in lithium-ion batteries and all-solid-state secondary batteries.
All-Solid-State Lithium Polymer
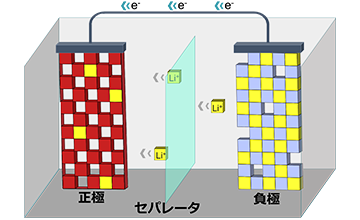
Existing lithium-ion batteries use flammable organic solvents, inherently carrying the risk of ignition due to liquid leakage. Therefore, all-solid-state lithium-ion batteries using non-flammable lithium-ion conductive polymer electrolytes have attracted attention. In this research, we are conducting molecular-level analysis of lithium-ion transport mechanisms, aiming to develop high-performance (high conductivity and high transport rate) electrolyte membranes.
CO2 Capture
Metal-Organic Frameworks (MOFs)

MOFs are porous materials with nanoscale pores obtained through the self-assembly of metal ions and organic ligands. They are expected to be applied in CO2 capture technologies for exhaust gases emitted from factories and power plants. The combination of metal ions and organic ligands allows for flexible design. We aim to develop new materials with high CO2 adsorption and long-term stability and propose theoretical design guidelines for MOFs with high CO2 adsorption and high stability using MD simulations.


